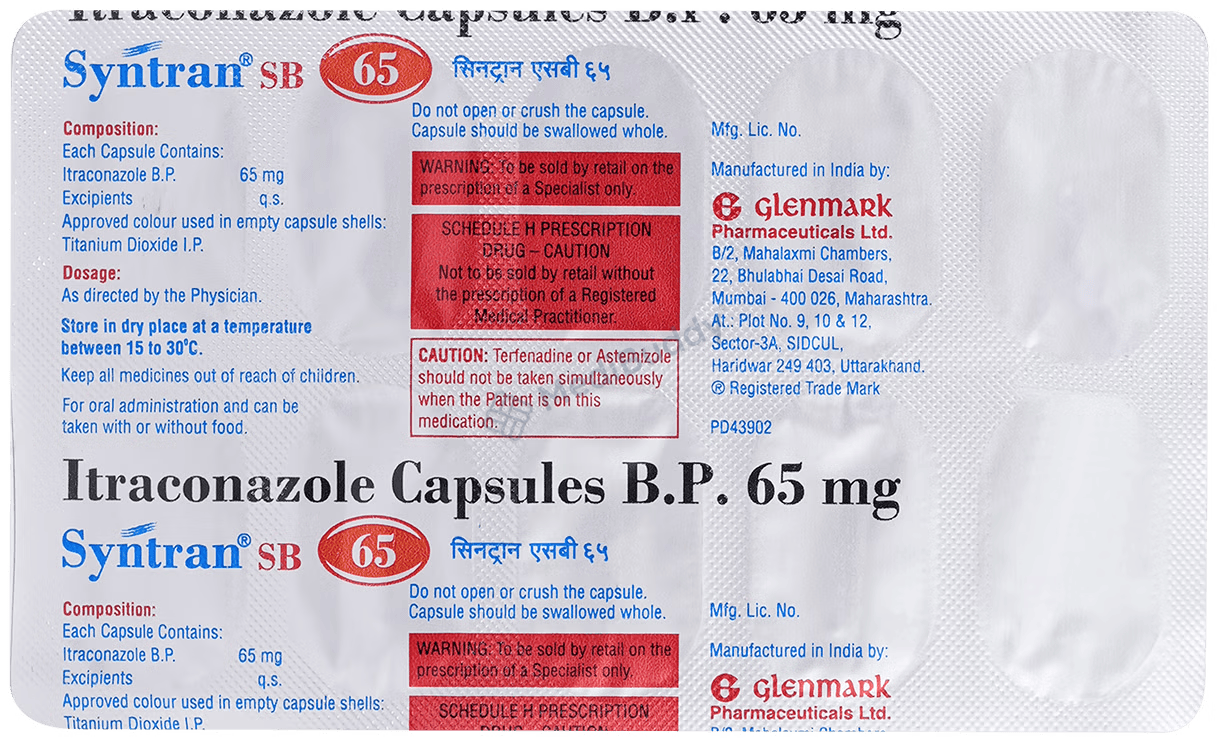
Syntran SB 65 Capsule
By Syntran
Rx
10 Capsule in a Strip

Composition
Itraconazole(65mg)

Manufacturer - Glenmark Pharmaceuticals Ltd
Glenmark Pharmaceuticals Limited, B/2, Mahalaxmi Chambers, 22, Bhulabhai Desai Road, Mumbai – 400 026.

Expires on or after
April, 2026

liver
Limited information is available regarding the use of Syntran SB 65 Capsule in patients with liver disease. It is essential to consult your doctor before starting this medication if you have any liver-related concerns. If you notice any symptoms of jaundice such as yellowing of eyes and skin, itching, or clay-colored stools while taking Syntran SB 65 Capsule, inform your healthcare provider promptly.

kidney
Syntran SB 65 Capsule needs caution in patients with kidney disease. Dose adjustment may be necessary. Please consult your doctor.

alcohol
When taking Syntran SB 65 Capsule, be cautious when drinking alcohol. Be sure to talk to your doctor about it.

driving
Syntran SB 65 Capsule may cause side effects such as dizziness, blurred/double vision, or hearing loss, affecting driving ability.

pregnancy
Syntran SB 65 Capsule may not be safe to use during pregnancy. Animal studies showed potential harm to the developing baby. Your doctor will assess the risks and benefits before prescribing. Please consult your doctor before using.

breastfeeding
Syntran SB 65 Capsule may not be safe during breastfeeding. Limited data indicates potential harm to the baby through breastmilk exposure.
| Habit Forming | No |
| Chemical Class | Azoles {Triazoles} |
| Therapeutic Class | ANTI INFECTIVES |
| Action Class | Fungal ergosterol synthesis inhibitor |
₹160
Inclusive of all taxes
Content verified by

Dr. Archana Prabhakar
MBBS, M.Med (Family Medicine)
Last update on 01-Oct-2024